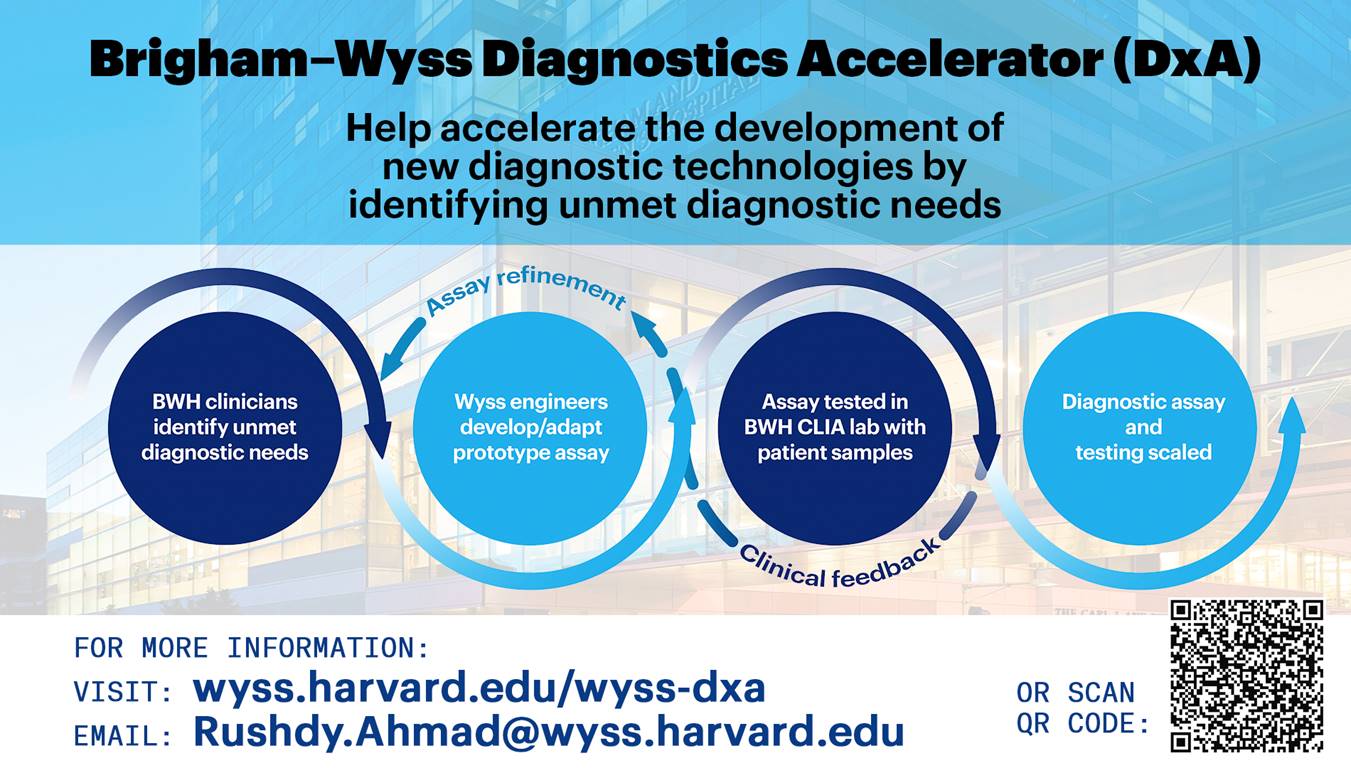
Brigham-Wyss Diagnostics Accelerator
Bench to Bedside: Clinical Collaboration
Our acceleration process begins with healthcare providers, researchers and administrators who identify critical unmet medical diagnostic needs. They then work alongside Wyss scientists and engineers to help develop technologies to address these specific problems. As promising technologies are developed in Wyss labs, they are brought to the CLIA-certified lab at the Brigham and Women’s Hospital (BWH) to be tested with real patient samples. The CLIA designation is granted by the FDA and sets clinical standards for clinical laboratory testing performed on human samples, which means these Wyss-developed technologies are being used under similar standards that the FDA uses when evaluating products for approval in the market. The Wyss teams then use clinician feedback to further refine and enhance these technologies before bringing them back to the CLIA lab.